Risk assessment principles
After identifying the potential occupational hazards and potential exposure routes, risk assessment must evaluate the likelihood of exposure, and the potential severity of harm should exposure occur.
Engaging and working with all stakeholders involved in the preparation and administration process is crucial when completing the risk assessment. This ensures that all perspectives and expertise are considered.
The risk assessment should be clearly documented by a trained and suitably experienced individual. A risk assessment template and some completed exemplar assessments are included below.
The Health and Safety Executive regulates health and safety at work and provides further detailed guidance on Control of Substances Hazardous to Health (COSHH) basics and principles of good control practice.
Risk assessment process
Risk can be represented as the equation:
Risk = hazard x probability of occurrence
In this context, “hazard” refers to the harm that might occur and “probability” refers to the likelihood that the harm will occur. Our article about occupational exposure to mAb (SPS page) provides more information about hazards and likelihood of exposure.
The aim is to reduce the risk as far as is reasonably practicable.
In most areas where mAb are handled, the appropriate risk mitigations are likely to be in place already. This is because these measures are often the same as those required for the handling of all injectable medicines.
There may not be a need to include further risk mitigation measures, if current practices are found to be satisfactory.
Risk assessment tool and worked examples
This risk assessment tool can be used to document the risk assessment
We include three worked examples, one of which is an antibody-drug conjugate to illustrate the difference between mAb and conjugated mAb.
Risk management and mitigation
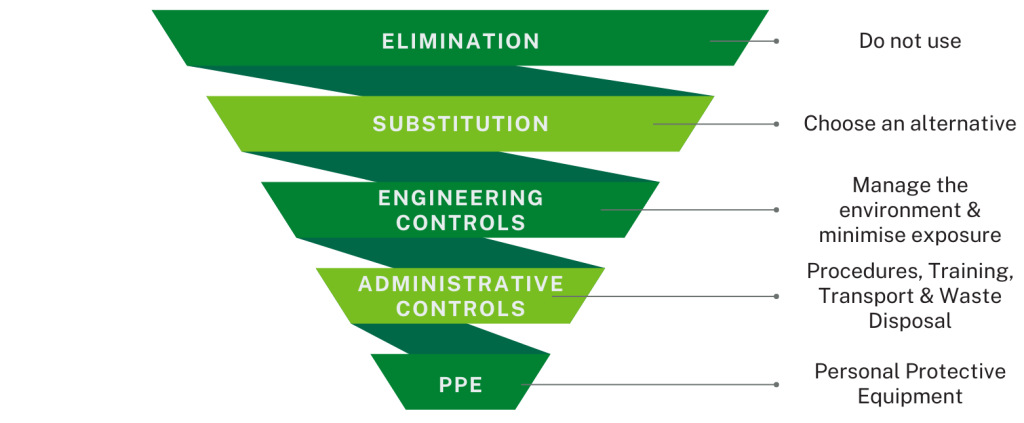
The hierarchy of controls (see diagram) provides a framework to identify and rank interventions. This assumes that general training for safe preparation and administration of injectable medicines is in place and delivered to all appropriate staff and that the associated routine levels of protection specified for all medicines administration are being followed.
Elimination
The most effective intervention is not to use the molecule. In some workplace settings this may be possible and achievable. This is not likely to be a practical solution in a healthcare setting where treatment choice is based on patient need.
Substitution
The next most effective control is to substitute the hazardous substance with an alternative molecule or presentation.
It is unlikely that the choice of mAb could be changed to one with lower risks, as the choice is based on the clinical need of the patient, However, a different formulation could be considered which may present lower preparation risks. For example, a sub-cutaneous injection is usually supplied ready-to-administer, while an IV infusion is likely to require further manipulations prior to administration.
Engineering Controls
In healthcare a more practical measure is to manage the environment and minimise the number of people exposed.
These could include, but are not limited to the following options.
Mitigations/controls to be considered include but are not limited to:
Environmental controls
Organisational policies for preparation and administration of injectable medicines may already require an appropriate workspace that allows safe preparation of medicines without distraction or risk of contamination to the product. This can also minimise the risk of spillages and needlestick injuries.
Isolators in pharmacy aseptic or radiopharmacy facilities
Monoclonal antibodies conjugated with cytotoxic agents or radioactive isotopes are specifically excluded from the following advice. These molecules should be handled according to relevant guidance and regulations using established controls, in pharmacy aseptic or radiopharmacy facilities.
Closed System Transfer Devices (CSTD)
The use of such devices is intended to mechanically prohibit the transfer of environmental contaminants into the system and therefore prevent the escape of the molecule outside of the system. There are no comprehensive, peer-reviewed studies to confirm their efficacy.
For those organisations choosing to use CTSD it is essential to ensure that users are appropriately trained in their use. Compatibility between drug and device and potential for drug loss during usage should also be considered. The use of CSTD within the pharmacy aseptic preparation process is outside the scope of this article.
Administrative Controls
This refers to controlling risks by the way people work.
Procedures
There should be clear processes and procedures available to staff to support safe handling of injectable medicines to protect both the patient and the staff, including prevention of
- errors
- contamination of the product
- aerosol formation
- contamination of the work area with spills.
These should be in date and easily accessible.
Training
Staff involved in mAb preparation and administration should be trained according to local policies and procedures on handling injectable medicines. Staff should also be aware of the key principles discussed in this article.
Transport and waste disposal
Procedures for the transport of mAb and the handling of waste should be guided by the molecule-specific risk assessment and local policies for the safe transport and disposal of medicines.
Use of Personal Protective Equipment (PPE)
Use of PPE is the least reliable option for controlling exposure to hazardous substances. It is important to remember that PPE should always be used with, not instead of, the risk controls described above, because of this.
Gloves worn for infection prevention reasons will also reduce the likelihood of skin contact with mAb, but may not be necessary.
Governance
NHS organisations should have an Injectable Medicines Policy, which may include mAb, or there may be a separate mAb policy.
The policy should clearly state the responsibilities of all staff involved in ensuring the safe handling of mAb within the organisation. As the professional lead for medicines within the organisation, the Chief Pharmacist should play a lead role in governance.
The organisational process and method of risk assessment of new and existing mAb therapies should be clearly documented, for example, included as part of the business process of a Medicines Management Committee Application or Drug & Therapeutics Committee.
The period for review of risk assessments should be clearly stated, including where the responsibility lies for ensuring the review is completed.
The policy should contain details of training required before staff are authorised to handle mAb; this may be mAb-specific training, or injectables medicines training already in use at the organisation.
Regular monitoring of the preparation process should be conducted to ensure adherence to Standard Operating Procedures (SOPs) and exposure controls. Any errors, incidents and non-compliances should be thoroughly documented and investigated according to local procedures.
Local decisions about the most appropriate location for preparation of mAb doses should take into account the potential risk to staff of occupational exposure as well as the patient-safety risks associated with dose preparation. A complex preparation process involving multiple vials and multiple manipulations would be most appropriately made in a pharmacy aseptic unit even if one or both of occupational exposure and patient-safety risks are low. Conversely a mAb requiring a simple draw up may best be made in a pharmacy aseptic unit if one or both categories of risk are considered to be elevated.
Update history
- Page title updated for clarity. Additional clarification added at the end of the Governance para.
- Published