Defining contamination
The European Union Guide to Good Manufacturing Practice (EUGMP) which remains applicable to medicines manufacturing and preparation in the UK defines contamination as “the undesired introduction of impurities of a microbiological nature (quantity and type of microorganisms, pyrogen), or of foreign particle matter, into or onto a raw material, intermediate, active substance or drug product during production, sampling, packaging or repackaging, storage or transport with the potential to adversely impact product quality”.
In aseptic manufacturing and preparation, the most likely potential sources of contamination include:
- micro-organisms and cellular debris (e.g. pyrogens, endotoxins) introduced from starting materials, the working environment and personnel
- particulates (e.g. glass and other visible and sub-visible particles) introduced during the manufacturing process
- chemical cross contamination introduced from residues on surfaces and airborne contaminants, such as from other medicines and cleaning or disinfection agents
Understanding contamination control
Contamination control is not a new concept. The principles of minimising the risk of contamination to medicinal products should already be core to any aseptic manufacturing or preparation service as described in section 3.5.19b of Guidance for ‘specials’ manufacturers.
Contamination control is achieved through effective implementation of a range of risk management measures, which collectively provide assurance of medicinal product quality and safety during the manufacturing or preparation process. These measures include:
- design, validation and control of facilities and equipment
- design and validation of processes, systems and procedures
- control of starting materials, components and packaging materials
- competent and knowledgeable personnel
- effective supervision, oversight and monitoring
- effective cleaning and disinfection
- a programme of quality risk management and continuous improvement
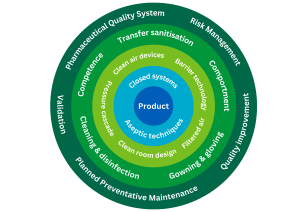
It may be helpful to view the aspects of contamination control as layers of protection for the product.
Changes to EUGMP
An update to EU GMP Annex 1 was published in August 2022 and remains applicable to the UK. Compliance was mandatory from August 2023. This requires all manufacturers of sterile medicines to have a Contamination Control Strategy (CCS).
The changes to EUGMP do not mean that existing contamination control systems must be replaced, but they should be reviewed and referenced in the CCS.
There is no explicit requirement for units operating under Section 10 exemption to document a CCS but they are advised to develop one to demonstrate that the risks to patients are fully understood effectively managed. Please note: Section 10 is an exemption from the requirement for an MHRA licence. It is not an exemption from the requirements of GMP.
Developing a Contamination Control Strategy
The CCS provides evidence that all risks to control of contamination are recognised and understood, and that appropriate monitoring and controls are in place. It ensures that all the inter-relationships between control measures are defined to facilitate investigation of deviations and out of specification results.
All these measures should already be in place in all NHS units, but may not be concisely and fully documented as a strategy. Development of the CCS therefore requires a good understanding of the principles of quality risk management, together with detailed technical knowledge of the facility, equipment, operational processes and quality system. Management responsibility for each aspect may be assigned to different individuals, so effective coordination is essential.
The strategy should explain or refer to:
- all the processes, facilities and equipment used to achieve asepsis and control all types of contamination
- how the processes are validated
- potential points of failure, and their significance
- the methods of monitoring the effectiveness of the controls including acceptance criteria
- action to be taken if acceptance criteria are not met
- how the strategy is used in practice to identify risks and to ensure continuous quality improvement
The principles and regulatory expectations for developing a CCS are detailed in EUGMP Annex 1.
Resources
This article will help you to plan and carry out a process risk assessment.
Templates and guides
A collaboration of SPS, The Pharmacy Aseptic Services Group (PASG), the NHS Pharmaceutical Quality Assurance Committee (PQAC) and other Pharmacy Quality Assurance (QA) colleagues, has produced the following series of resources to support development of a local CCS for aseptic services. These resources may be adapted for local use when developing a site-specific CCS. The template for MS units is applicable to traditional sterile manufacturing units.