Presentation
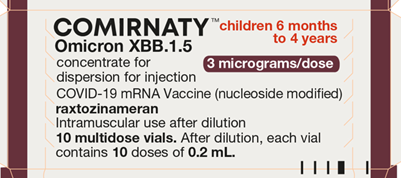
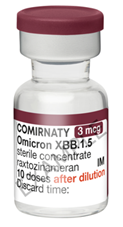
Comirnaty Omicron (XBB.1.5) 3 micrograms/dose concentrate for dispersion for injection COVID-19 mRNA Vaccine (nucleoside modified) raxtozinameran is presented in multi-dose vials for dilution with 2.2mL 0.9% sodium chloride solution for injection. We refer to it as Comirnaty 3 (THREE) (XBB.1.5) concentrate in our web articles and SOPs.
Comirnaty 3 (THREE) (XBB.1.5) concentrate is thawed by the NHS’s Specialist Pharmaceutical Logistics (SPL) providers before supply to the NHS. The SPLs supply either thawed original cartons each containing 10 multidose vials or packed down cartons containing 2 vials.
The thaw label applied by SPLs bears the new shelf life at 2-8°C. The vials inside the cartons are not labelled with the post thaw expiry date so it is essential that the vials remain within their carton until the point of removal from the fridge for immediate use.
Carton / Vial Artwork
Carton
Vial
Consumables and Patient Information Leaflets
Patient Information Leaflets, and syringes and needles for dilution and administration are provided with the vaccines.
Shelf life and storage
Undiluted vaccine
Comirnaty 3 (THREE) (XBB.1.5) concentrate may be stored for up to 10 weeks at 2-8°C following thawing.
Within the 10 weeks, it may spend up to 24 hours in total at room temperature (between 8ºC and 30°C) following removal from the refrigerator. This permitted time/conditions includes up to 12 hours of storage following first puncture.
Diluted vaccine
Once diluted, the vaccine vial may be stored for up to 12 hours at room temperature (up to 30°C). This must be within the allowed use period of 10 weeks at 2°C to 8°C and 24 hours at 8°C to 30°C.
However, from a microbiological point of view, unless the method of dilution precludes the risk of microbial contamination, the product should be used as soon as practically possible e.g. within one work session which would not normally be more than 6 hours.
The manufacturer’s summary of product characteristics contains additional information on the handling of temperature excursions.
Shelf life extensions
From time to time, variations to the marketing authorisation may mean that some batches may have shelf lives beyond the labelled manufacturer’s expiry date. If there are any applicable shelf life extensions, they will be listed on our vaccine expiry extension page.
Transport and movement
Undiluted vaccine
Undiluted vaccine vials stored at 2-8°C may be transported throughout their 10 week post-thaw shelf life.
Further information can be found on our Transport of COVID-19 vaccines article and our Mutual Aid guidance.
Diluted vaccine
The diluted product may be transported for up to 6 hours at room temperature. However, the vaccines contain no preservative and the method of dilution cannot preclude the risk of microbial contamination, so SPS advises that transport of diluted vials should not be routine.
The decision to move diluted Comirnaty 3 (THREE) (XBB.1.5) concentrate between locations within the same legal entity must include an assessment of the risk of microbial contamination and proliferation versus risk of wastage and loss of opportunities to administer vaccines at alternative locations.
Any decision to move punctured vials must be made locally under the direction of the Chief Pharmacist or site lead pharmacist, taking the specific circumstances into account, and using appropriate risk control measures such as temperature control, infection prevention and control, and a means to identify that the vial has been diluted.
Preparation
After dilution with 2.2mL 0.9% sodium chloride solution for injection, each Comirnaty 3 (THREE) (XBB.1.5) concentrate vial contains 10 doses.
Different presentations of the Comirnaty vaccine may not require dilution, or may require the use of different diluent volumes. Caution is required to ensure that the correct dilution method is followed for the presentation being used.
Detailed advice about preparation, including a model standard operational procedure can be found on our Preparing Comirnaty 3 (THREE) (XBB.1.5) concentrate vaccine page.
Risk management
Comirnaty 3 (THREE) (XBB.1.5) concentrate will be handled in the same location as other vaccines. Following the guidance on Handling multiple COVID-19 Vaccines will reduce the risk of errors.
Licensed status
Comirnaty 3 (THREE) (XBB.1.5) concentrate has a full Marketing Authorisation (PLGB) in the UK.
Vaccine vials being supplied to the UK have been labelled for the European market and so bear an EU marketing authorisation number rather than the PLGB number, and have one or more EU Patient Information Leaflets (PILs) in the carton. These vials are still considered licensed in the UK, but the EU PILs should not be given to patients. GB PILs are provided with the vaccines.
Allergy, excipients and dietary advice
Comirnaty 30 (XBB.1.5) is contraindicated in those with a hypersensitivity to the active substance or to any excipient (ingredient) in the vaccine, although revaccination may be possible on the advice of an expert (e.g. allergy specialist). See the Summary of Product Characteristics (SPC) for full details of the active substance and excipients included in the vaccine.
Allergy to any excipient is possible, but polyethylene glycol (PEG) is of particular importance. See the Green Book Chapter 14a: COVID-19 for details on how to manage individuals with a history of allergy.
Comirnaty 30 (XBB.1.5) is free from gluten, nut, soy, latex and animal products such as egg. However, as with most pharmaceutical products the manufacturer cannot guarantee that minute amounts of substances (listed previously) are not contained in raw materials from their suppliers, or that the product has not come into contact with latex during the manufacturing process.
Useful resources
Refer to the following recommended resources for further information.
Summary of Product Characteristics
Manufacturer’s Supporting Information
Update history
- Published